Gore Ventures
Internal Ventures
Innovation Approach
Gore’s Internal Ventures group is comprised of wholly-owned, organically derived “startup” teams that leverage lean innovation approaches – where Associates rapidly test, learn and validate or invalidate opportunities for potential commercial success. Validated concepts may become part of an existing Gore business if they fit with their growth strategy, a new commercial business for Gore, or an external venture.
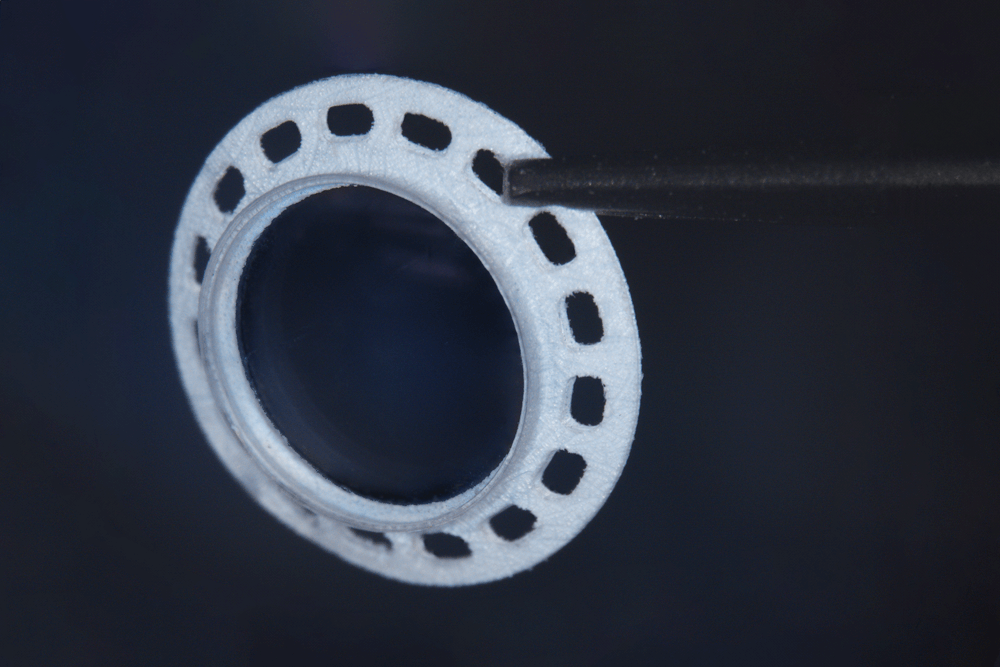
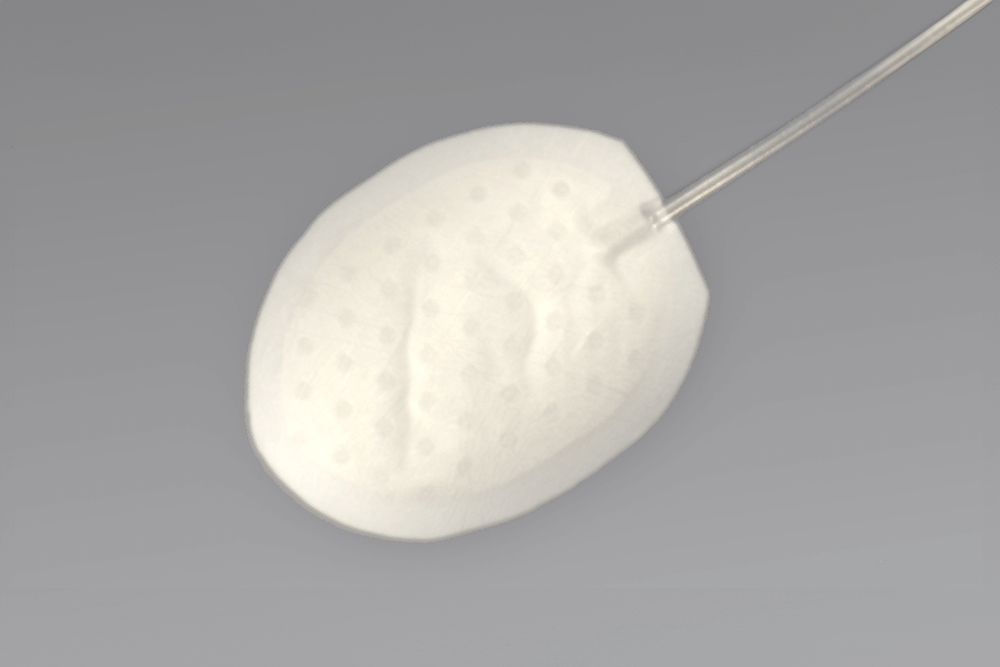
Pursuing a Viable Alternative for Corneal Tissue Transplants
Gore is developing an investigational synthetic cornea device with goal of providing an alternative treatment for corneal opacity - the 4th leading cause of blindness worldwide.1 The soft, transparent fluoropolymer optic combined with a porous, biocompatible expanded PTFE (ePTFE) skirt is intended to restore vision when donor corneas fail.
1 Murthy_GVS, Johnson_GJ. Prevalence, incidence and distribution of visual impairment. In: The Epidemiology of Eye Disease. 3rd ed. Imperial College Press, 2012;1:3-61
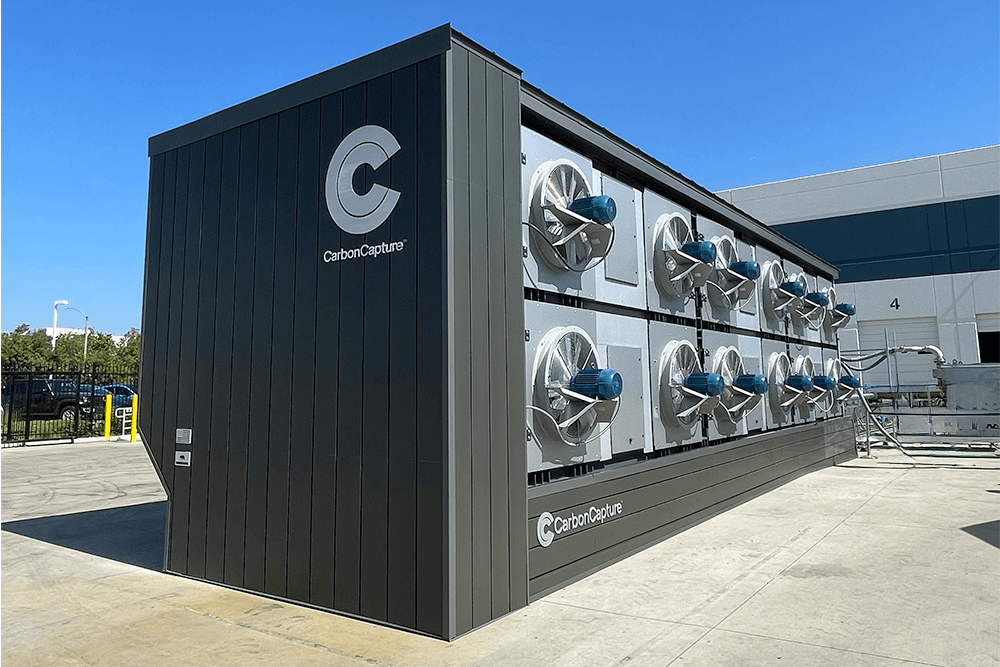
Paving the Way for Cleaner Air
Gore’s Structured Direct Air Capture (DAC) Contactor, used in systems that remove carbon dioxide from the atmosphere, minimizes pressure drop and increases steam stability of the sorbent material.
Gore’s composite structures enable and enhance a variety of sorbent technologies, creating scalable solutions to advance DAC technology.
Envisioning the Future of Glaucoma Treatment
Gore is developing an investigational implant intended to treat glaucoma, a condition that damages the optic nerves and can lead to blindness. Often caused by persistent high pressure in the eye, a glaucoma drainage implant reduces the pressure by draining the accumulated fluid. However, with conventional implants, the eye tissue that absorbs the fluid can scar over time, reducing efficacy.
Our porous, biocompatible ePTFE reservoir incorporated in this investigational device is intended to minimize fibrosis a with a goal of improved durability.
Internal Ventures News & Events
Membrion secures $20 million to lead the future of industrial water circularity
Series B1 round anchored by Pangaea Ventures, PureTerra Ventures, Ecolab Inc., and W. L. Gore & Associates fuels expansion of sustainable wastewater treatment at commercial scale
- / News
Our Active Membership in CarbonTech Leadership Council and Carbon to Value (C2V) Initiative
The Carbontech Leadership Council (CLC) is an invitation-only group of corporate, non-profit, and government thought leaders who foster commercialization opportunities and identify avenues for technology validation, testing, and demonstration.
- / News
What is New in Artificial Corneas
Recently, reinvigorated efforts in developing fully synthetic flexible devices have resulted in several prototypes now undergoing clinical trials.
- / News
COFEPRIS Approves Clinical Trial for GORE Synthetic Cornea
The Federal Commission for Protection against Sanitary Risks (COFEPRIS) has recently authorized a pioneering clinical trial, which aims to assess the effectiveness of the GORE synthetic cornea device in patients suffering from corneal clarity loss.
- / Events
Exciting Clinical Trial Data at AGS 2025
Join Dr. Juan Batlle at the AGS 2025 Annual Meeting for a presentation on the Gore investigational device, its design, materials and early clinical results on sub-conjunctival scarring, a key cause of filtering surgery failure.
Mitico Secures $4.3 Million Seed Funding to Commercialize Industrial Carbon Capture Technology
Mitico, a pioneering carbon capture technology company, today announced it raised $4.3 million in seed funding to scale its innovative granulated metal carbonate sorption technology capturing carbon dioxide (CO2) emitted from industries. The round led …