Gore Ventures
Investigational Glaucoma Drainage Implant
Exploring innovative surgical treatments for the world’s leading cause of blindness.
Developing an Investigational Glaucoma Drainage Device for Patients Who Require Surgical Treatment
- Using a stable, biopermeable interface design intended to provide durable pressure reduction to treat moderate-to-severe glaucoma
- Developed in collaboration with leading ophthalmologists and glaucoma specialists
- Fully enrolled feasibility studies are ongoing
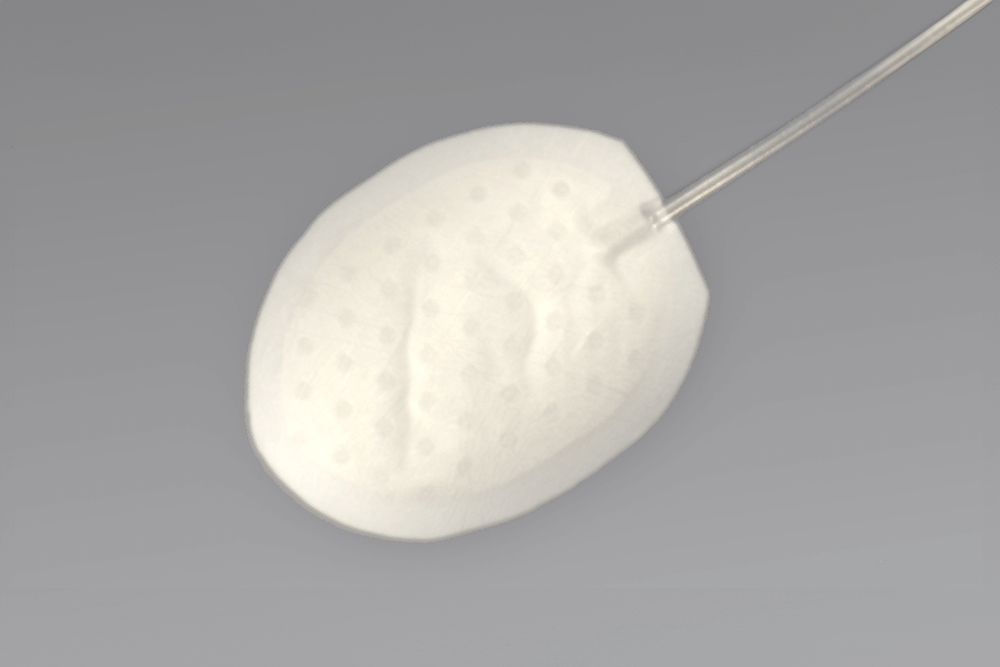
Tackling the Leading Cause of Blindness
Glaucoma affects an estimated 4.2 million people in the U.S., making it the most common cause of irreversible blindness. Without treatment, elevated eye pressure can damage the optic nerve, leading to vision loss1.
In early stages, elevated intraocular pressure is managed with daily topical medications. However, poor adherence—often due to medication side effects, complex dosing regimens, cost, and forgetfulness—can lead to disease progression, necessitating surgical intervention in moderate-to-severe cases2.
Surgery is typically a last resort due to significant risks and a high failure rate—up to 50% within five years—after other lower-risk but less effective treatments have been exhausted2.
Seeking a New Solution for Pressure Relief
We are investigating innovative and novel materials and designs that could benefit patients with moderate-to-severe glaucoma by providing an alternative to traditional tube shunt devices.
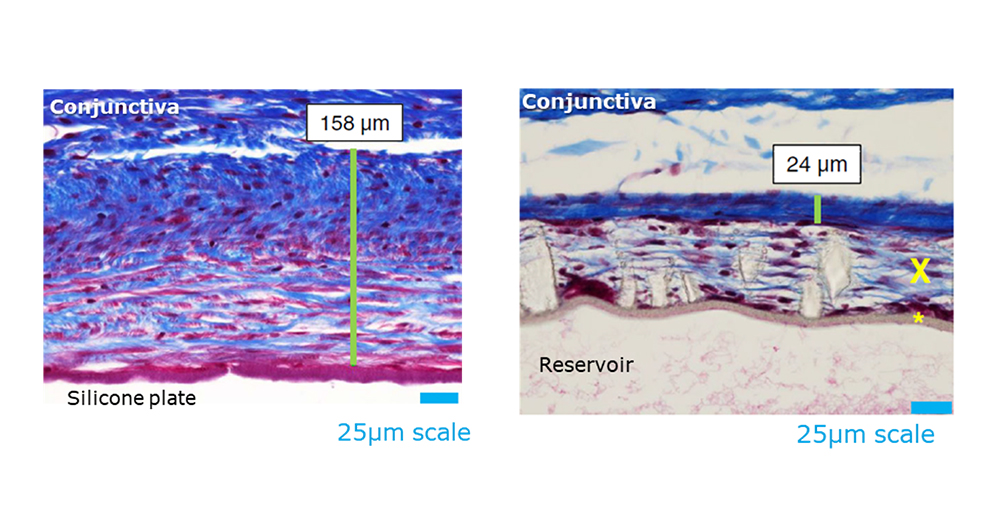
The implant features a unique reservoir design and specialized material that may reduce fibrosis without requiring antifibrotic drugs during implantation. Additionally, its design is intended to minimize the bleb profile, potentially improving patient comfort and clinical outcomes.
Product Resources
References
- Ehrlich, J.R., Burke-Conte, Z., Wittenborn, J.S., Saaddine, J., Omura, J.D., Friedman, D. S., Flaxman, A.D., & Rein, D.B. (2024). https://www.cdc.gov/vision-health-data/prevalence-estimates/prevalence-estimates-glaucoma.html. JAMA Ophthalmology.
- Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Survey of ophthalmology. 2008;53(6):S57-S68.Chan, Lilian, Marlene R. Moster, Amanda K. Bicket, Arsham Sheybani, Steven R. Sarkisian, Thomas W. Samuelson, Iqbal Ike K. Ahmed, Eydie Miller-Ellis, Oluwatosin U. Smith, and Qi N. Cui. “New Devices in Glaucoma.” Ophthalmology and Therapy, August 9, 2023. https://doi.org/10.1007/s40123-023-00780-3.
- Bicket, Amanda Kiely, Julia Szeto, Peter Roeber, Jeff Towler, Mitch Troutman, E Randy Craven, Anup Khatana, et al. “A Novel Bilayered Expanded Polytetrafluoroethylene Glaucoma Implant Creates a Permeable Thin Capsule Independent of Aqueous Humor Exposure.” Bioengineering & Translational Medicine, 2020. https://doi.org/10.1002/btm2.10179.
- Bicket, Amanda Kiely, and Ian Pitha. “OVERCOMING BLEB ENCAPSULATION WITH BIOMATERIALS.” Glaucoma Today, 2022.
Latest News
- / Events
Exciting Clinical Trial Data at AGS 2025
Join Dr. Juan Batlle at the AGS 2025 Annual Meeting for a presentation on the Gore investigational device, its design, materials and early clinical results on sub-conjunctival scarring, a key cause of filtering surgery failure.
- / Study
GORE Glaucoma Drainage Implant Clinical Study Dominican Republic
The objective of this early feasibility clinical study is to evaluate the safety and effectiveness of the GORE Glaucoma Drainage Implant (2 configurations) in subjects with primary open-angle glaucoma that is uncontrolled by hypotensive medications or for which conventional incisional glaucoma surgery would be more likely to fail due to scarring.
- / News
Overcoming Bleb Encapsulation With Biomaterials
An investigational integrated glaucoma drainage implant prototype can create thinner, more permeable blebs.